Understanding South Africa’s biodiversity through DNA barcoding
South Africa’s rich biodiversity spans terrestrial, freshwater, and marine ecosystems, encompassing complex community assemblages of organisms. This diversity is a crucial national asset, underpinning strategic objectives across conservation, sustainable resource management, and socio-economic development1. Understanding and documenting biodiversity is therefore essential for informed decision-making, guiding conservation strategies, sectoral policies, and sustainable use of our natural resources to address challenges such as poverty, inequality and unemployment2.
Despite this, many South African ecosystems and species remain understudied, even as they face growing pressures like habitat loss and fragmentation, unsustainable use, climate change, biological invasions and illegal trade. For numerous species, conservation status remains data deficient or unassessed, complicating efforts to evaluate and mitigate threats. These knowledge gaps are compounded by incomplete taxonomic data, including uncertainties in species classification, identification and distribution across bioregions.
An important tool that can assist in addressing these challenges is DNA barcoding, which enables rapid and accurate species identification using standardised genetic markers3,4. DNA barcoding improves understanding of species diversity across ecosystems and biomes5,6, supports the updating of national species checklists, and helps fill taxonomic gaps in priority groups. It complements traditional taxonomy by reliably delimiting species and identifying Operational Taxonomic Units (OTUs), including cryptic, closely related or morphologically similar populations7,8. DNA barcoding also has practical applications in environmental DNA (eDNA) monitoring of ecosystems, tracking invasive species9–11 and forensic identification of illegal wildlife trade in South Africa12,13.
DNA barcoding relies on short, standardised DNA regions for identification – e.g., mitochondrial cytochrome oxidase subunit I (COI) for many animals, maturase K (matK0 and ribulose-1,5-bisphosphate carboxylase/oxygenase (RbcL) for plants, and Internal Transcribed Spacer (ITS) for fungi13.
South African Barcode of Life (SABOL)
The South African Barcode of Life (SABOL) is the national node of the International Barcode of Life Consortium (iBOL). SABOL is funded by the Department of Science, Technology and Innovation (DSI) through the Foundational Biodiversity Information Programme (FBIP). SABOL promotes DNA barcoding research, addressing gaps in species reference libraries, and consolidates scattered biodiversity data into one comprehensive national resource. DNA barcoding strengthens biodiversity assessments, particularly for cryptic and understudied taxa.
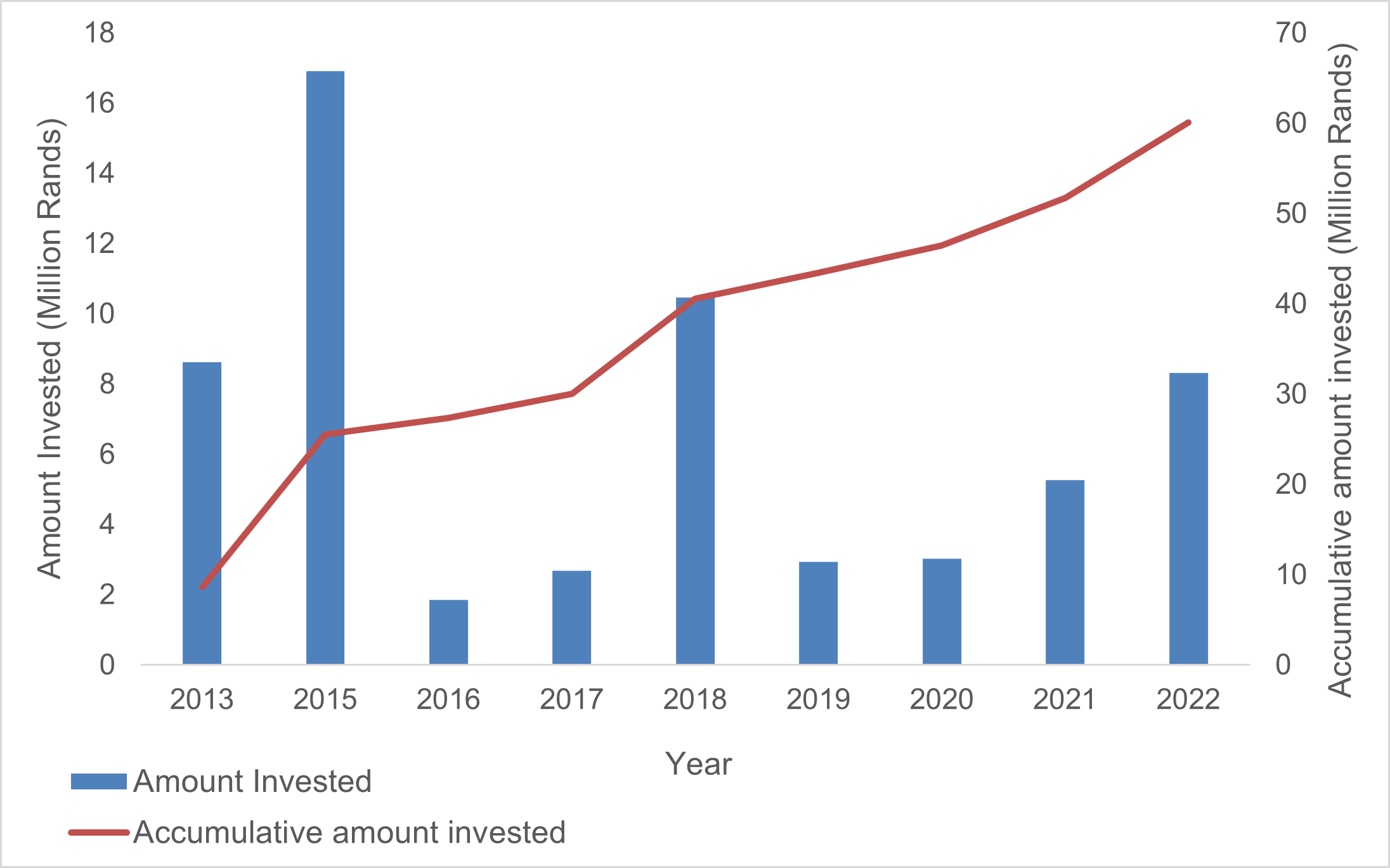
Between 2013 and 2022, the FBIP funded 124 DNA barcoding-related projects totalling over R60 million (Figure 1). These efforts align with both international and national obligations, including the United Nations Convention on Biological Diversity (CBD), the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES), and South Africa’s National Environmental Management: Biodiversity Act (NEM:BA).
A key component of DNA barcoding is the availability of a comprehensive reference library of expertly identified specimens. The Barcode of Life Database (BOLD) serves as a trusted repository for these sequences, ensuring that barcodes meet rigorous quality standards and are derived from taxonomically verified specimens. By providing a reliable foundation for species identification, BOLD underpins SABOL and other national barcoding initiatives, enabling accurate biodiversity assessments and monitoring.
South African barcoding efforts to date
Over the past decade, South Africa has made significant progress in expanding its faunal DNA barcoding database, particularly for insects and other invertebrates. A 2016 review of DNA barcoding reported that South Africa had contributed approximately 48 000 faunal DNA barcodes to BOLD, representing approximately 2.3% of all known species globally, at the time14.
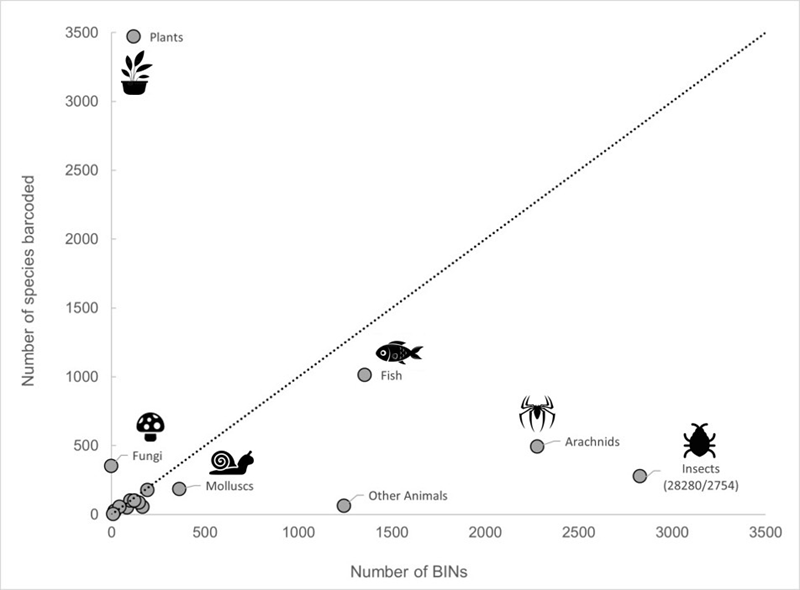
Since 2016, contributions to the reference library have grown substantially, with numerous South African researchers and institutions submitting data on indigenous species. As of 28 October 2025, BOLD contains 786 480 sequences (multiple gene sequences for some records), representing 776 512 specimens from South African localities. Approximately, 93% (725 590) of these records are assigned to 59 606 Barcode Index Numbers (BINs), which represent OTUs. However, only 94 010 specimen records have been identified to species level, representing 16 425 species (Figure 2). This highlights ongoing needs for taxonomic capacity and expertise.
This milestone reflects the collaborative efforts of 284 research institutions, demonstrating the commitment of South Africa’s biodiversity research community to building a comprehensive DNA barcode species reference library. Despite this progress, many of the same challenges first documented nearly a decade ago in the national DNA barcoding review14 remain, including incomplete geographical coverage, taxonomic gaps, limited taxonomic expertise, and the underutilisation of barcode data in conservation and management frameworks14,15.
Future directions and recommendations
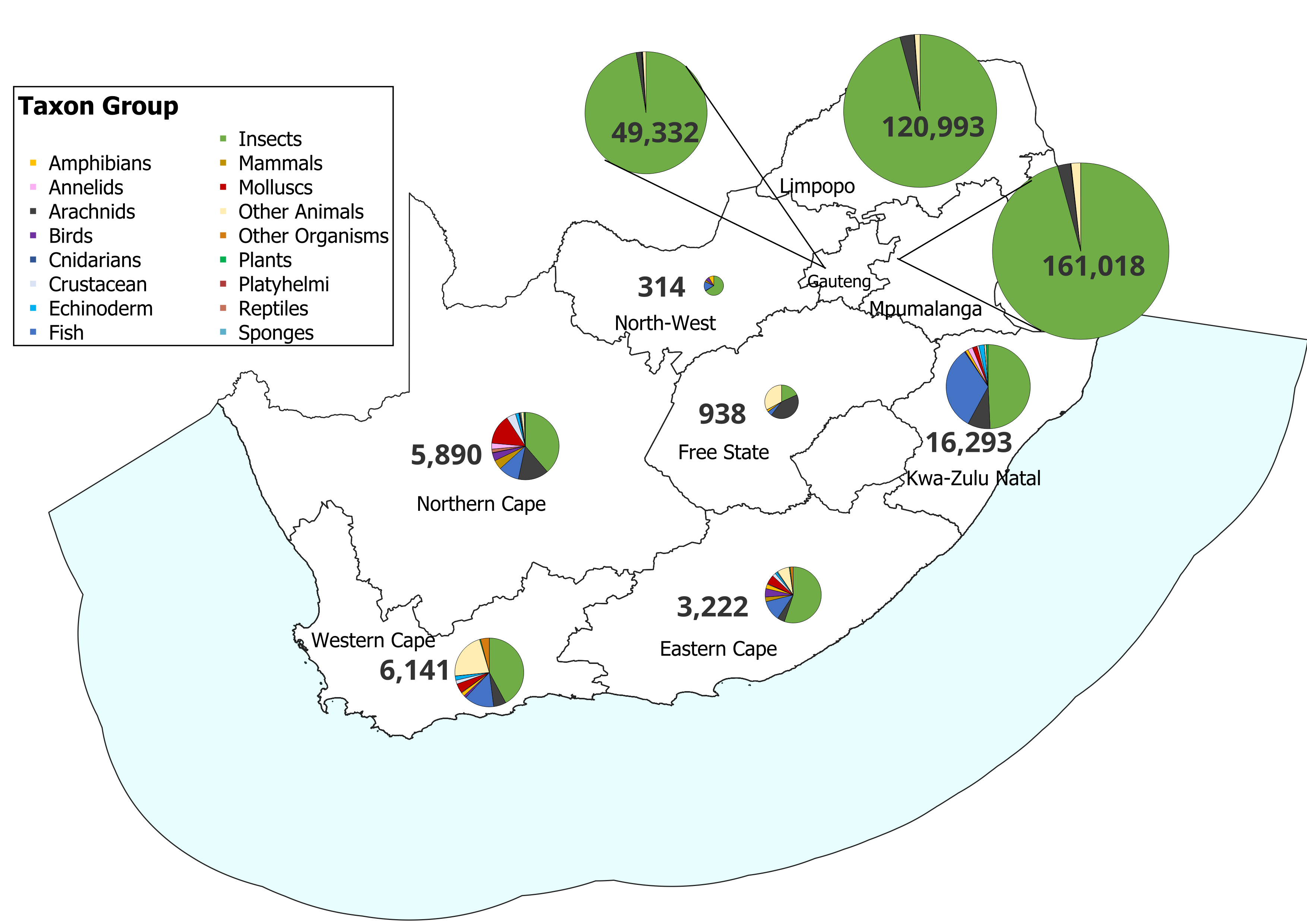
A 2025 gap analysis15 indicates that South Africa’s reference library still has taxonomic and geographic gaps, particularly in priority taxa and under-sampled regions (Figure 3). The BIN system relies on multiple sequences per species that capture intraspecific variation across distributions and to improve phylogenetic resolution7. To maximise its effectiveness, future sampling should focus on:
entire species’ ranges, especially for known species that have not yet been barcoded;
sparsely sampled areas, including biodiversity hotpots and threatened ecosystems;
understudied taxonomic groups, ensuring priority taxa are adequately represented.
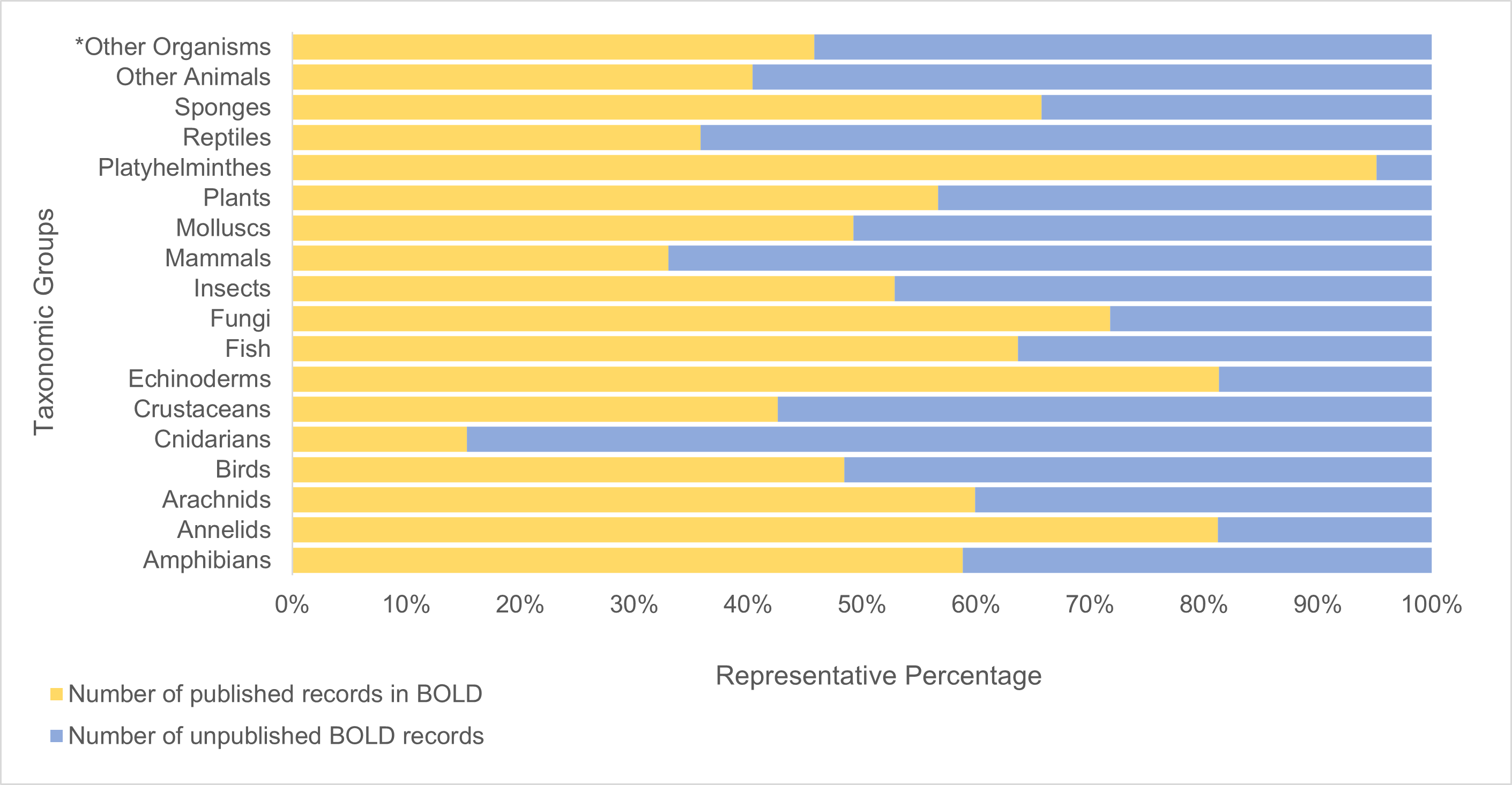
Currently, a significant number of South African records on BOLD remain private while researchers analyse them (Figure 4). While understandable, this can create redundancies and slow the growth of the publicly accessible reference library.
To address these challenges, it is recommended that DNA barcoding efforts be coordinated and collaborative, ensuring national resources are used efficiently and research gaps are strategically targeted. Establishing a directory of existing barcoding projects and project leaders, alongside regular SABOL-led community meetings and conferences, will strengthen communication and collaboration among institutions.
Recommended citation
Kgatla, M., Baxter, J., & Mwale, M. 2025. DNA barcoding in South Africa: Genetic diversity tools. National Biodiversity Assessment 2025. South African National Biodiversity Institute. http://nba.sanbi.org.za/.